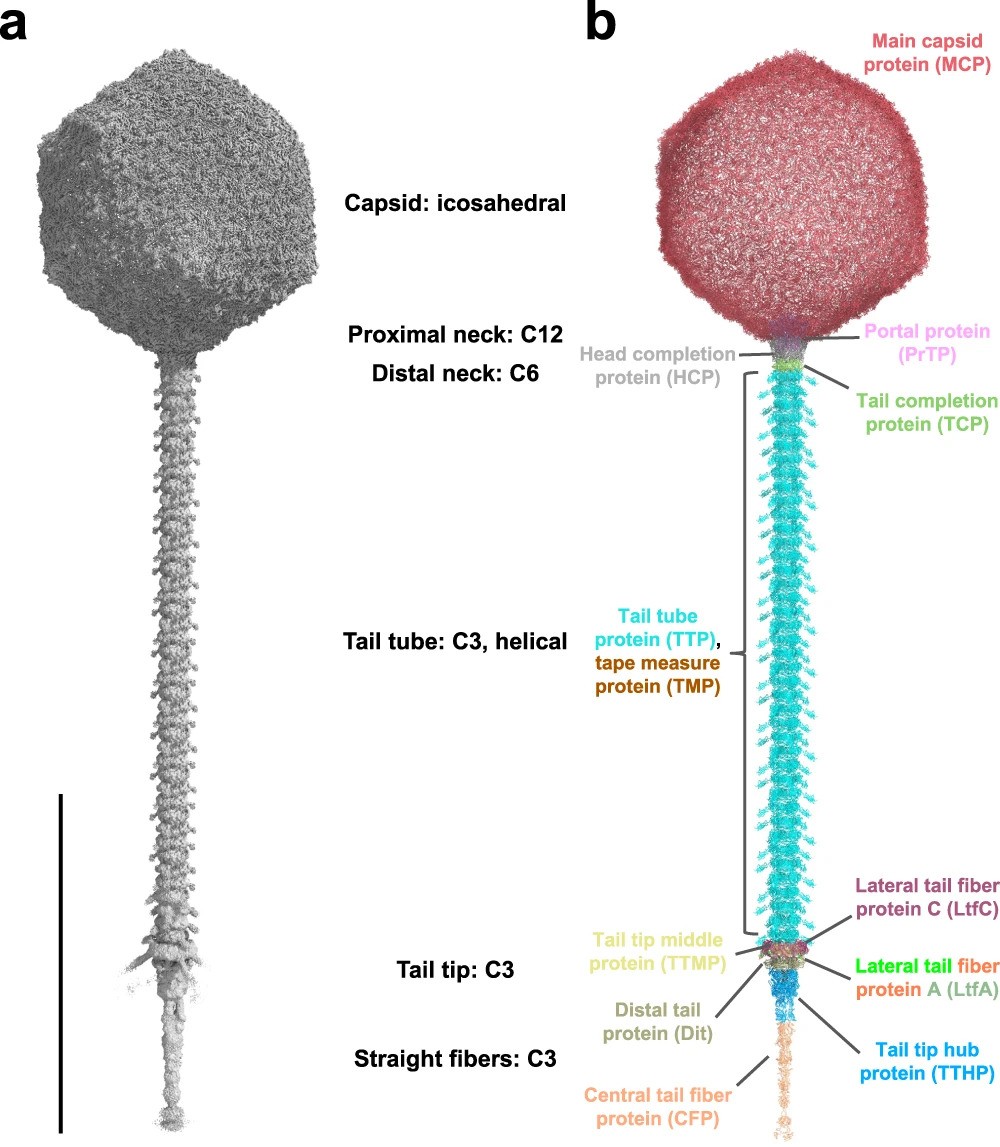
Architecture of the DT57C bacteriophage.
Scientists have unraveled the intricate molecular structure of bacteriophage DT57C, a member of the T5 family of tailed bacteriophages. These viruses are characterized by a long non-contractile tail and play a vital role in controlling bacterial populations. The DT57C bacteriophage, closely related to the well-known T5 phage, exhibits unique characteristics, including recognition of a distinct receptor (BtuB) and highly divergent lateral tail fibers (LTF).
Using advanced cryo-electron microscopy techniques, researchers obtained a comprehensive atomic model of the DT57C virus. This detailed structural analysis allowed for a deeper understanding of its organization and function. Notably, the study revealed novel features such as the mechanism of lateral tail fiber attachment, facilitated by a dodecameric collar protein (LtfC), and the composition of the phage neck, which consists of three protein rings.
Of particular interest was the arrangement of the tape measure protein (TMP) within the tail tube—a three-stranded parallel α-helical coiled coil that directly interacts with the viral DNA. Remarkably, the presence of the C-terminal fragment of TMP within the tail tip suggests a mechanism for the restoration of the tail tip complex after DNA ejection, shedding light on the process of infection and replication.
These findings not only provide a complete atomic structure of a T5-like phage but also offer insights into fundamental aspects of viral biology, including the process of DNA ejection. Furthermore, the structural details uncovered in this study lay the groundwork for the design of engineered phages with enhanced therapeutic potential and pave the way for future mechanistic investigations.
The development of new methods to tackle the challenges associated with visualizing complex viral structures demonstrates the interdisciplinary nature of this research and its potential impact on various fields, including phage therapy, gene therapy, and bioremediation. By elucidating the molecular architecture of bacteriophage DT57C, scientists have opened doors to a deeper understanding of viral biology and the potential applications of these viruses in biotechnology and medicine.
Image Description:
a Iso-electron potential surface of a composite cryo-EM map of the entire virion. Six individually symmetrized focused maps (containing capsid, portal, distal neck, tail, tail tip and straight fiber regions) were reconstructed at resolutions between 2.9–4.9 Å and combined into a composite map (see Supplementary Information and Methods). The normalized (average density 0, standard deviation 1) composite map was contoured at 3.0σ above average.
b Refined atomic model of the entire phage DT57C in ribbon representation. A total of 12 gene products are distinguished by colors which are consistently used throughout the manuscript. The scale bar represents a length of 1000 Å.
Photo credits: Ayala, R., Moiseenko, A.V., Chen, TH. et al. Nat Commun 14, 8205 (2023).
