Exploring the Phageome: Differences in Healthy and Atopic Dermatitis Skin
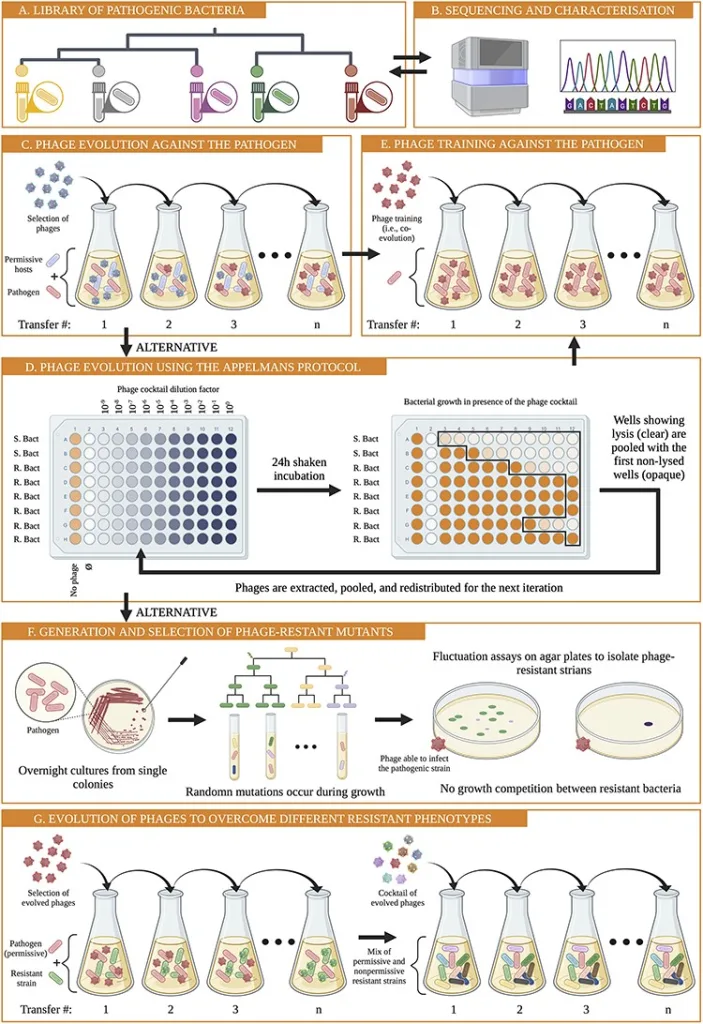
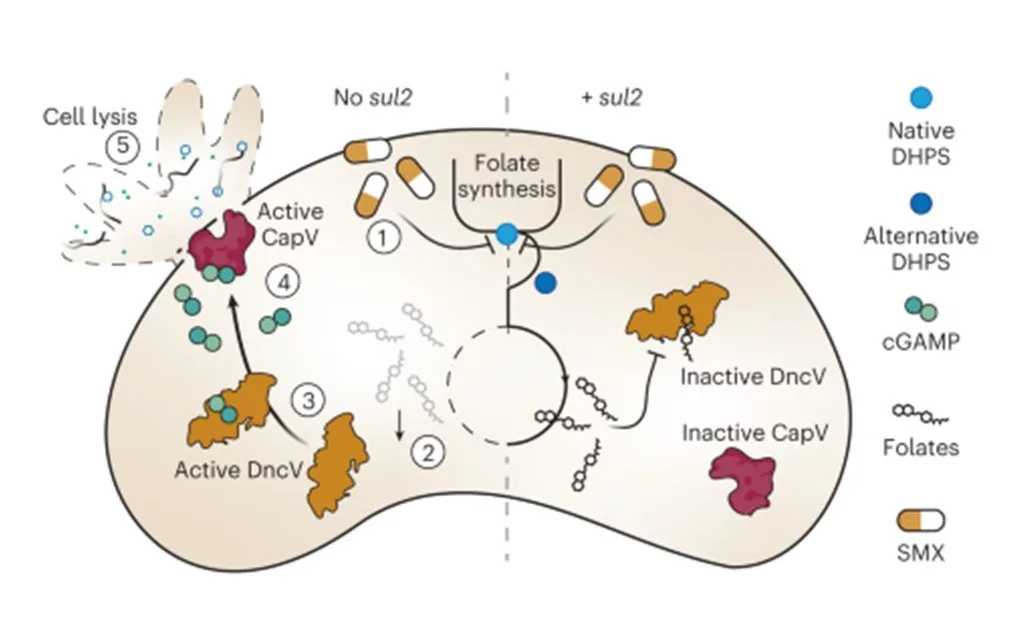
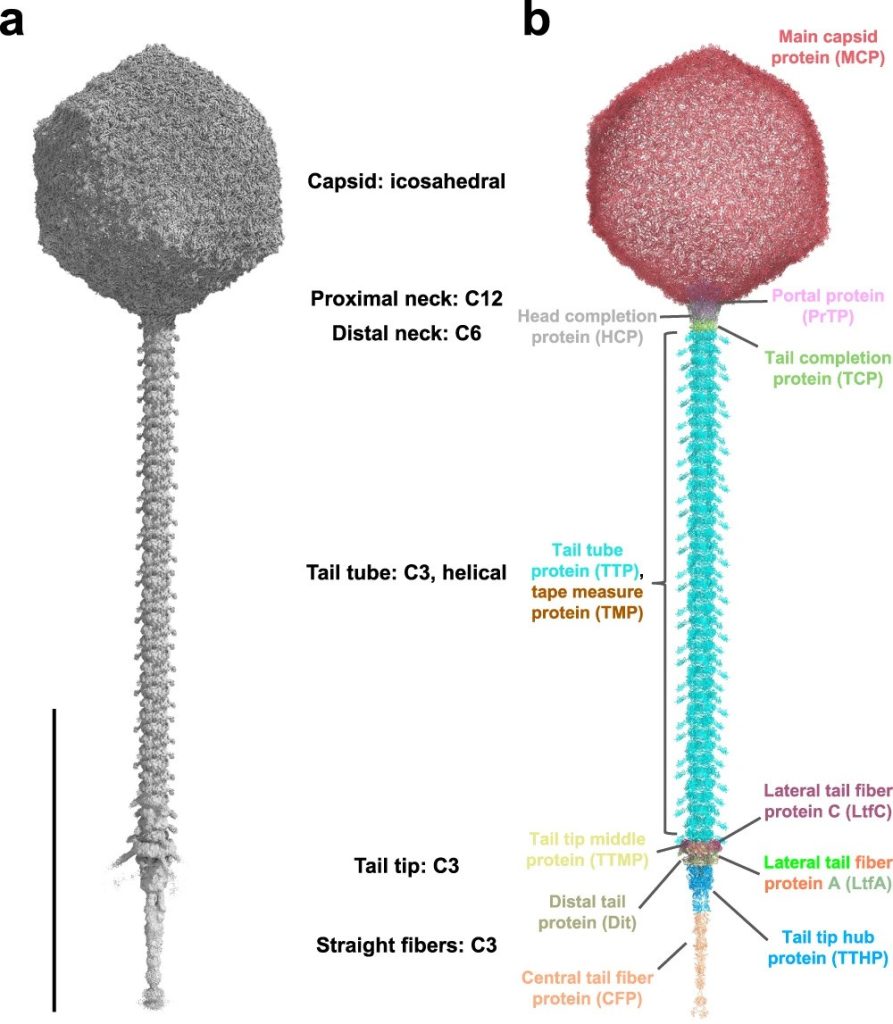
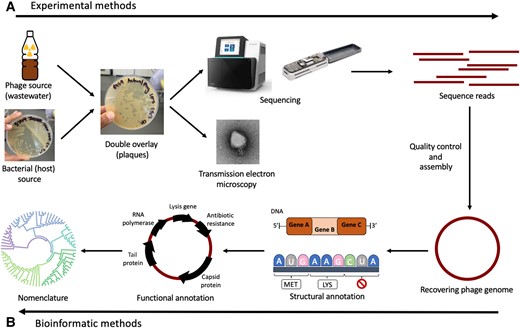
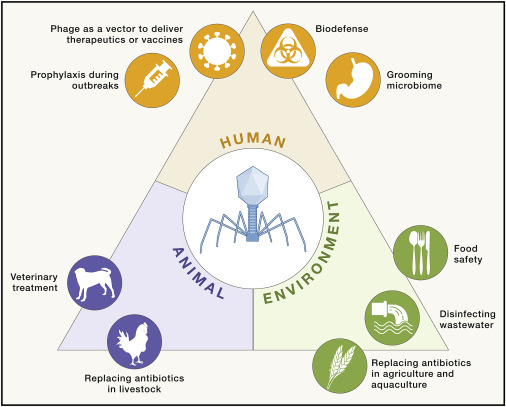
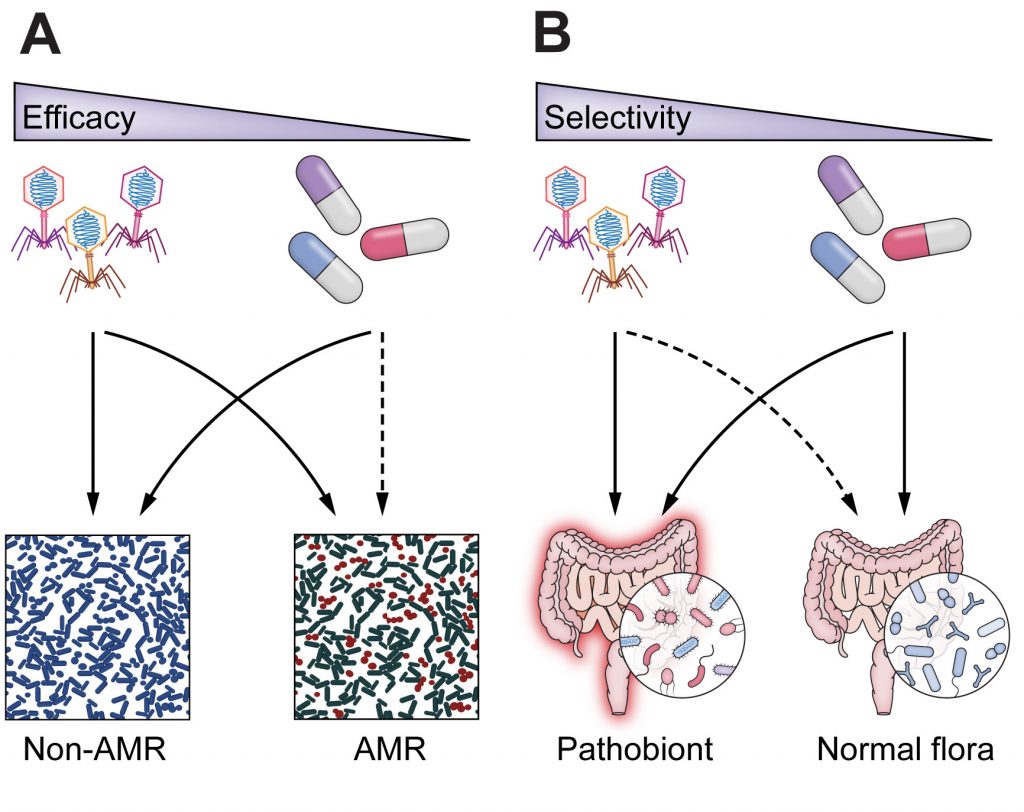
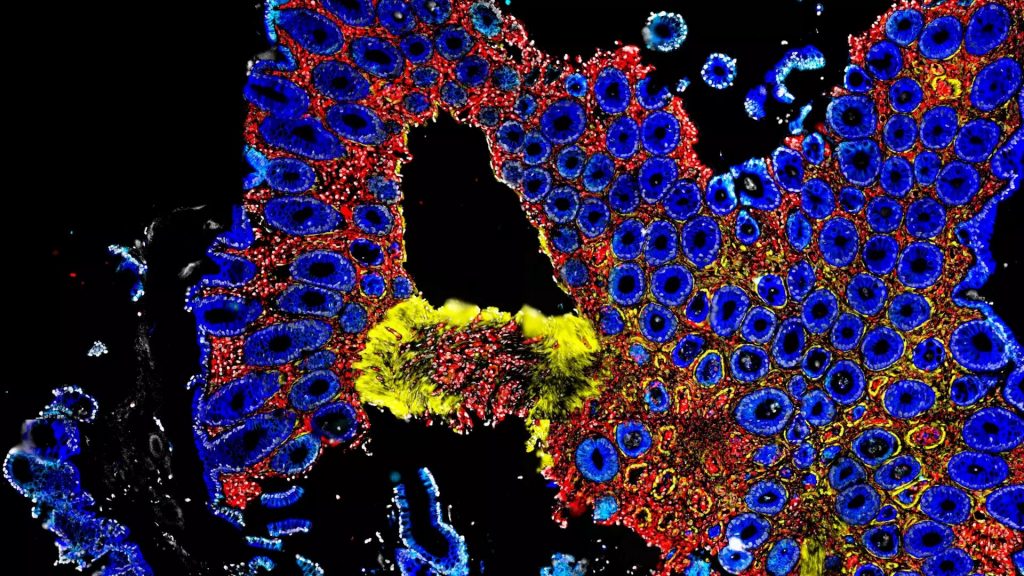
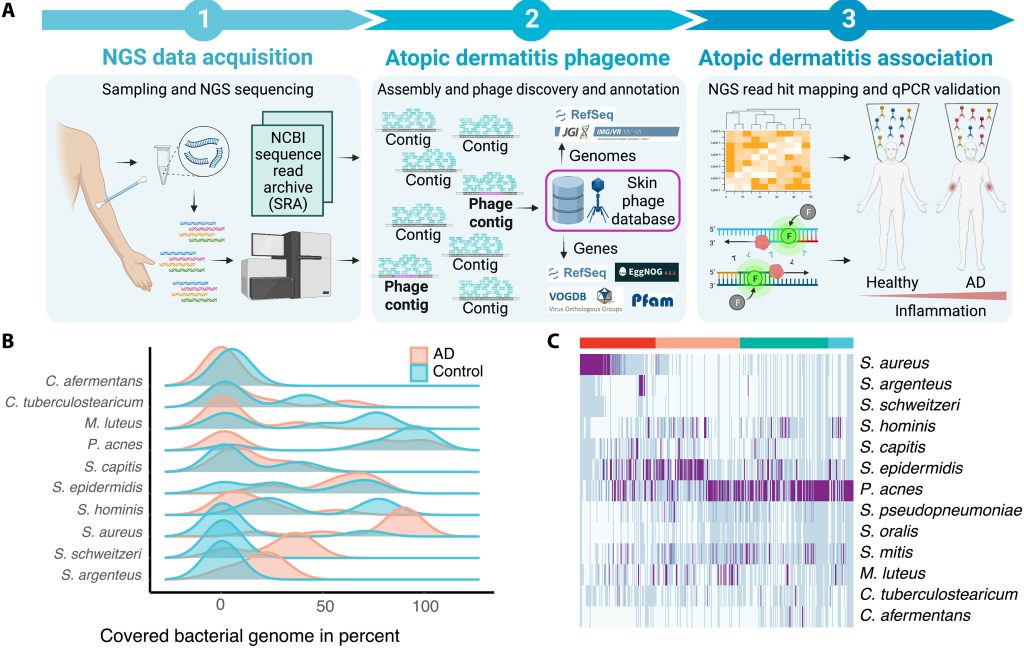
Study workflow and bacterial communities in healthy skin and AD. In this study published in Science Advances, Wolfgang Weninger and his team from the Medical University of Vienna, have highlighted the role of bacteriophages in the skin’s microbiome, particularly in relation to atopic dermatitis (AD). By analyzing skin swabs from both healthy individuals and AD…