On-target antifolate activity is essential for CBASS–antifolate interaction in V. cholerae.
A recent study led by Ana Rita Brochado, published in Nature Microbiology, shed light on the modulation of resistance and killing by antifolate antibiotics through the Vibrio cholerae CBASS phage defense system.
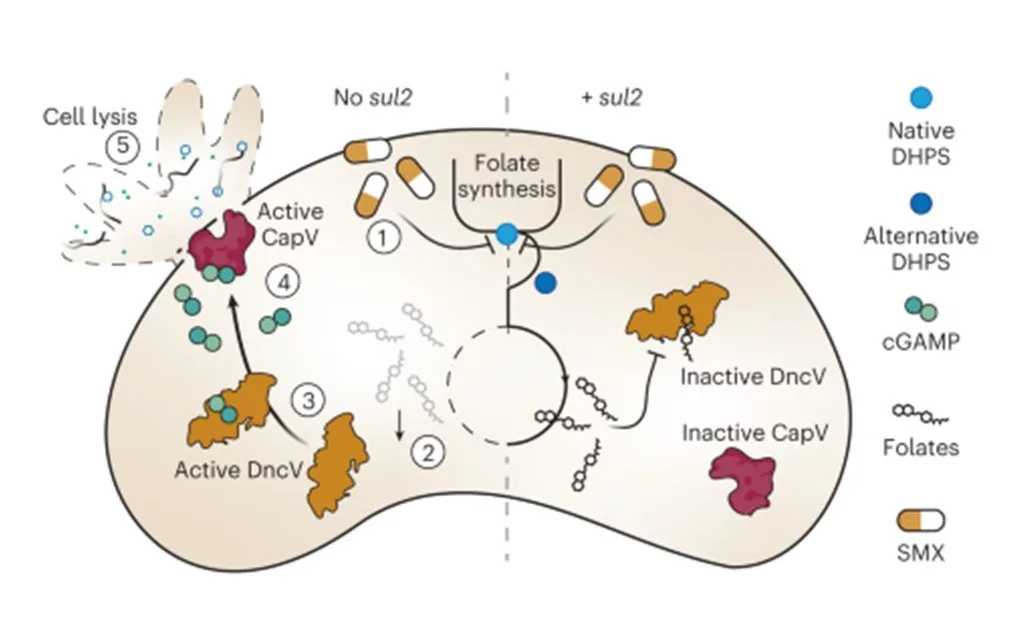
In their research, Brochado and her team, discovered that the cyclic-oligonucleotide-based anti-phage signaling system (CBASS) in Vibrio cholerae increases sensitivity to antifolate antibiotics by up to 10 times. This system also interferes with the synergy of these antibiotics, ultimately leading to bacterial lysis by these classic bacteriostatic antibiotics.
The study revealed that cyclic-oligonucleotide production by the CBASS nucleotidyltransferase DncV upon antifolate treatment confirms full CBASS activation under these conditions. It suggests that antifolates release DncV allosteric inhibition by folates. Moreover, the interaction between CBASS and antifolates is specific to CBASS systems with closely related nucleotidyltransferases and similar folate-binding pockets.
Additionally, the research found that antifolate resistance genes nullify the CBASS-antifolate interaction by bypassing the effects of on-target antifolate activity. This creates potential for their coevolution with CBASS.
In summary, the findings illustrate how toxic modules such as CBASS can influence antibiotic activity, ultimately conferring bactericidal activity to classical bacteriostatic antibiotics. This discovery offers new insights into antibiotic resistance mechanisms and potential avenues for the development of novel antimicrobial strategies.
Photo Credits: Brenzinger, S., Airoldi, M., Ogunleye, A.J. et al. Nat Microbiol 9, 251–262 (2024).
