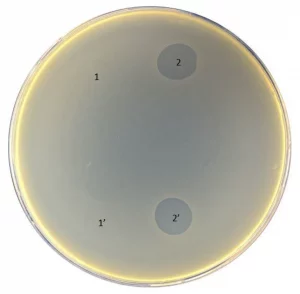
Pyobacteriophag effect before (1 and 1′) and after (2 and 2′) adaptation to strain 01206UR
Escherichia coli is a common cause of biofilm-associated urinary tract infections. Bacteria inside the biofilm are more resistant to antibiotics. In a study by Mukane et al, six E. coli strains isolated from patients with urinary tract infections were screened for biofilm-forming capability and antimicrobial susceptibility.
Two of the most significant biofilm-producing strains were selected for minimal inhibitory concentration and minimal biofilm eradication concentration in vitro testing using amoxicillin–clavulanic acid, ciprofloxacin, and three commercial bacteriophage cocktails (Pyobacteriophag, Ses, and Intesti). In case of a low phage effect, an adaptation procedure was performed.
Although the biofilms formed by strain 021UR were resistant to amoxicillin–clavulanic acid and ciprofloxacin, the three phage cocktails were able to reduce biofilm formation. In contrast, phages did not affect the 01206UR strain against planktonic and biofilm-forming cells. After Pyobacteriophag adaptation, the effect improved, and, regardless of the concentration, the adapted phage cocktail could destroy both planktonic cells and the biofilm of strain 01206UR.
Thus, bacteriophages capable of killing bacteria in biofilms can be used as an alternative to antibiotics. However, each case should be considered individually due to the lack of clinical trials for phage therapy. Antimicrobial and phage susceptibility should be determined in biofilm models before treatment to achieve the desired anti-biofilm effect.
© Image – Mukane et al.
Targeting Phage Therapy 2023 will highlight the current and potential anti-biofilm applications of phage therapy. Abstracts about this topic can be submitted until before the beginning of May. Abstract Submission Process.
Targeting Phage Therapy 2023 Congress
6th World Conference
June 1-2, 2023 – Paris, France
