Typhoid-conjugate vaccines (TCVs) provide an opportunity to reduce the burden of typhoid fever, caused by Salmonella Typhi, in endemic areas. As policymakers design vaccination strategies, accurate and high-resolution data on disease burden is crucial. However, traditional blood culture-based surveillance is resource-extensive, prohibiting its large-scale and sustainable implementation.
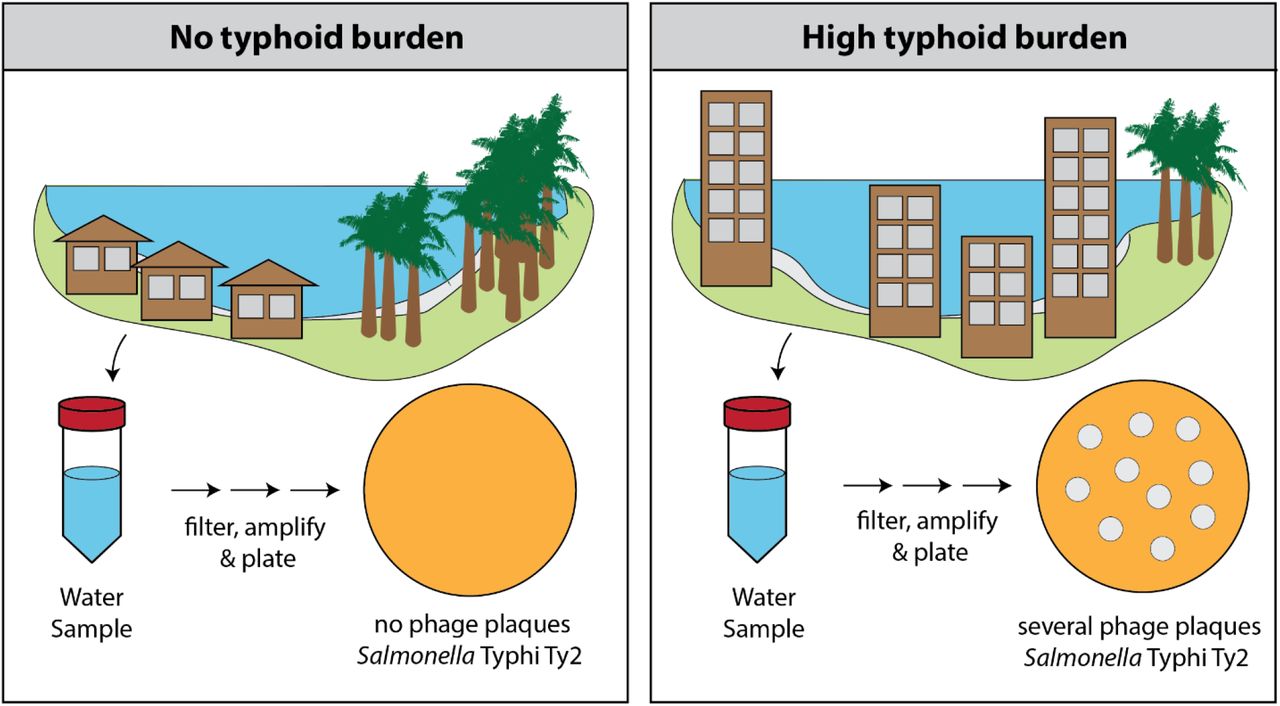
Salmonella Typhi is a water-borne pathogen, and here, Hooda et al. tested the potential of Typhi-specific bacteriophage surveillance in surface water bodies as a low-cost tool to identify where Salmonella Typhi circulates in the environment.
In 2021, water samples were collected and tested for the presence of Salmonella Typhi bacteriophages at two sites in Bangladesh: urban capital city, Dhaka, and a rural district, Mirzapur. Salmonella Typhi-specific bacteriophages were detected in 66 of 211 (31%) environmental samples in Dhaka, in comparison to 3 of 92 (3%) environmental samples from Mirzapur. In the same year, 4,620 blood cultures at the two largest pediatric hospitals of Dhaka yielded 215 (5%) culture-confirmed typhoid cases, and 3,788 blood cultures in the largest hospital of Mirzapur yielded 2 (0.05%) cases. 75% (52/69) of positive phage samples were collected from sewage.
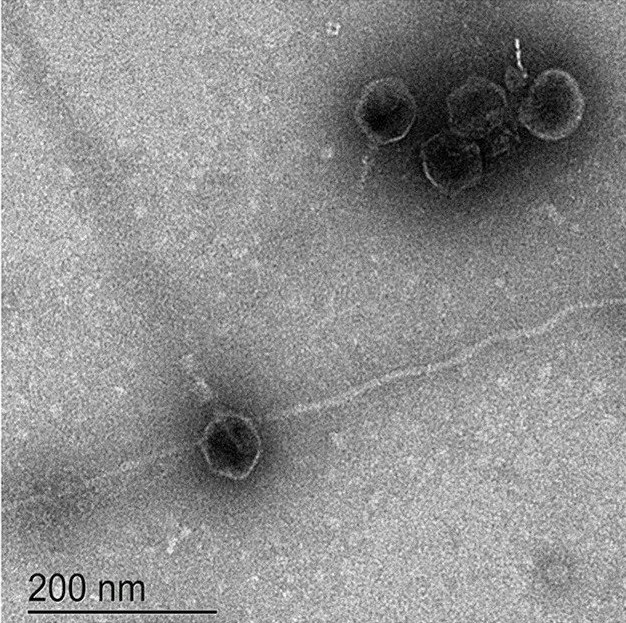
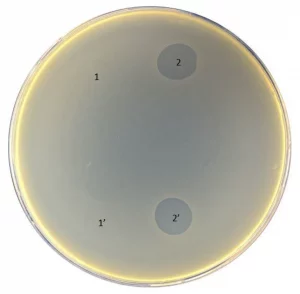
All isolated phages were tested against a panel of isolates from different Salmonella Typhi genotypes circulating in Bangladesh and were found to exhibit a diverse killing spectrum, indicating diverse bacteriophages were isolated.
These results suggest an association between the presence of Typhi-specific phages in the environment and the burden of typhoid fever, and the potential of utilizing environmental phage surveillance as a low-cost tool to assist policy decisions on typhoid control.
Highlights of the study
-
Typhi-specific bacteriophages can be isolated from surface waters in endemic countries using low-cost methods
-
More Typhi-specific bacteriophages are obtained in areas with higher typhoid cases
-
Typhi-specific bacteriophages exhibit diverse activity spectrum against a panel of Salmonella Typhi isolates circulating in Bangladesh
-
Environmental surveillance can be used as a tool to predict typhoid burden
Targeting Phage Therapy 2023 will elaborate on the latest phage discoveries. Learn about the program.
Targeting Phage Therapy 2023 Congress
6th World Conference
June 1-2, 2023 – Paris, France