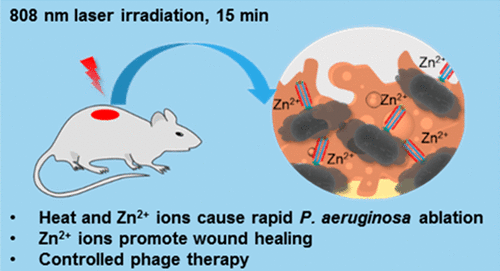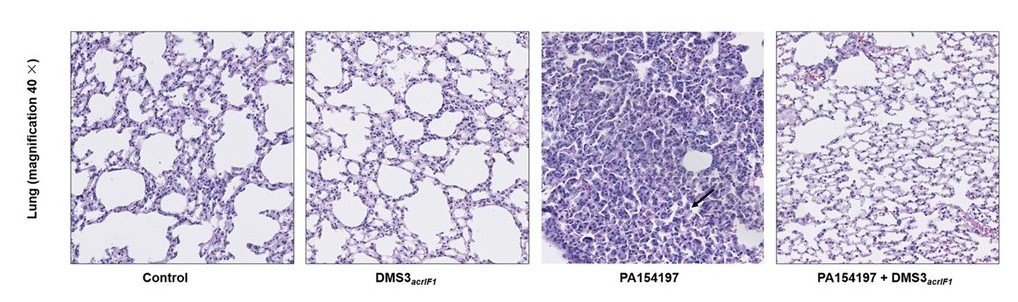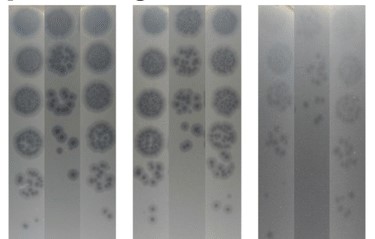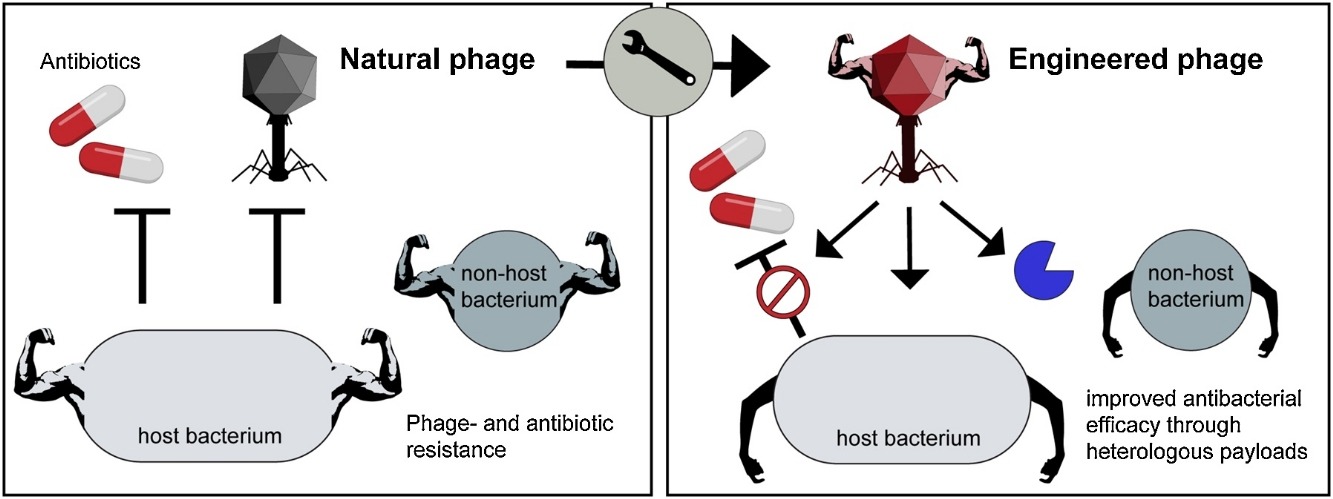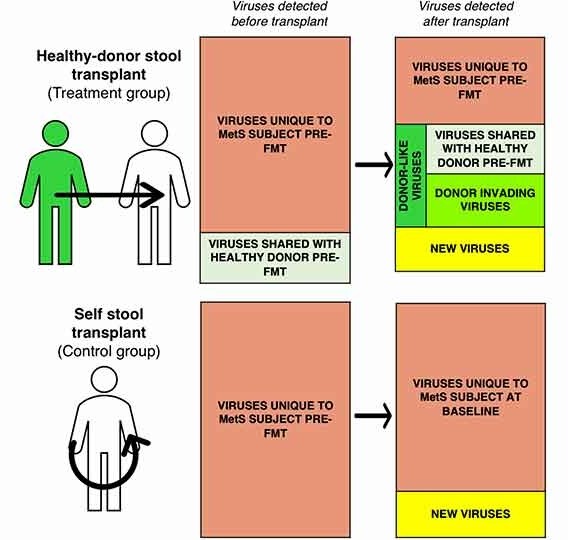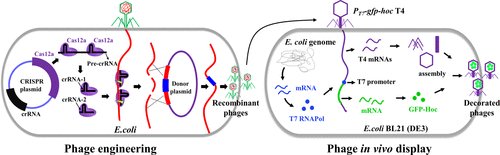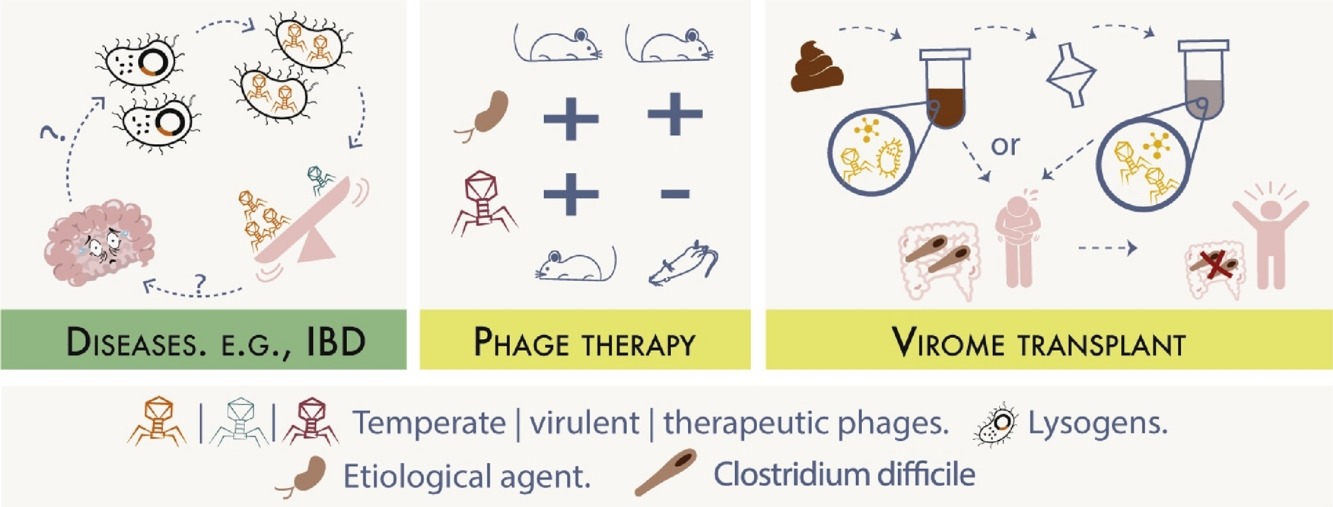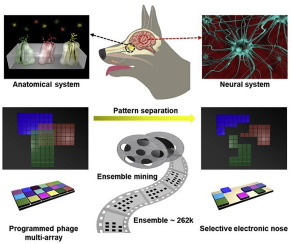
Increasing evidence suggests that gut dysbiosis is associated with coronavirus disease 2019 (COVID-19) infection and may persist long after disease resolution. The excessive use of antimicrobials in patients with COVID-19 can lead to additional destruction of the microbiota, as well as to the growth and spread of antimicrobial resistance.
The problem of bacterial resistance to antibiotics encourages the search for alternative methods of limiting bacterial growth and restoring the normal balance of the microbiota in the human body. Bacteriophages are promising candidates as potential regulators of the microbiota.
In their recent study, Zurabov et al. used two complex phage cocktails targeting multiple bacterial species in the rehabilitation of thirty patients after COVID-19. They also evaluated for the first time the effectiveness of the bacteriophages against the clinical strain of Klebsiella pneumoniae, using real-time visualization on a 3D Cell Explorer microscope.
The researchers had interesting results:
- Application of phage cocktails for two weeks showed safety and the absence of adverse effects.
- An almost threefold statistically significant decrease in the anaerobic imbalance ratio, with an erythrocyte sedimentation rate (ESR).
This work will serve as a starting point for a broader and more detailed study of the use of phages and their effects on the microbiome.
Targeting Phage Therapy 2023 will introduce the latest advances on phages & microbiota during the 6th World Conference on June 1-2. You can also submit an abstract concerning this subject.
Targeting Phage Therapy 2023 Congress
6th World Conference
June 1-2, 2023 – Paris, France